

The reason behind this can be stated simply as keeping them in the main table would make the periodic table too wide. The lanthanides (atomic numbers 57 to 71) and actinides (atomic numbers 89 to 103) come in separate rows in the periodic table. It has since been extended with the discovery of new metals, with the table remaining extremely useful in the field of chemistry as well as other scientific fields.
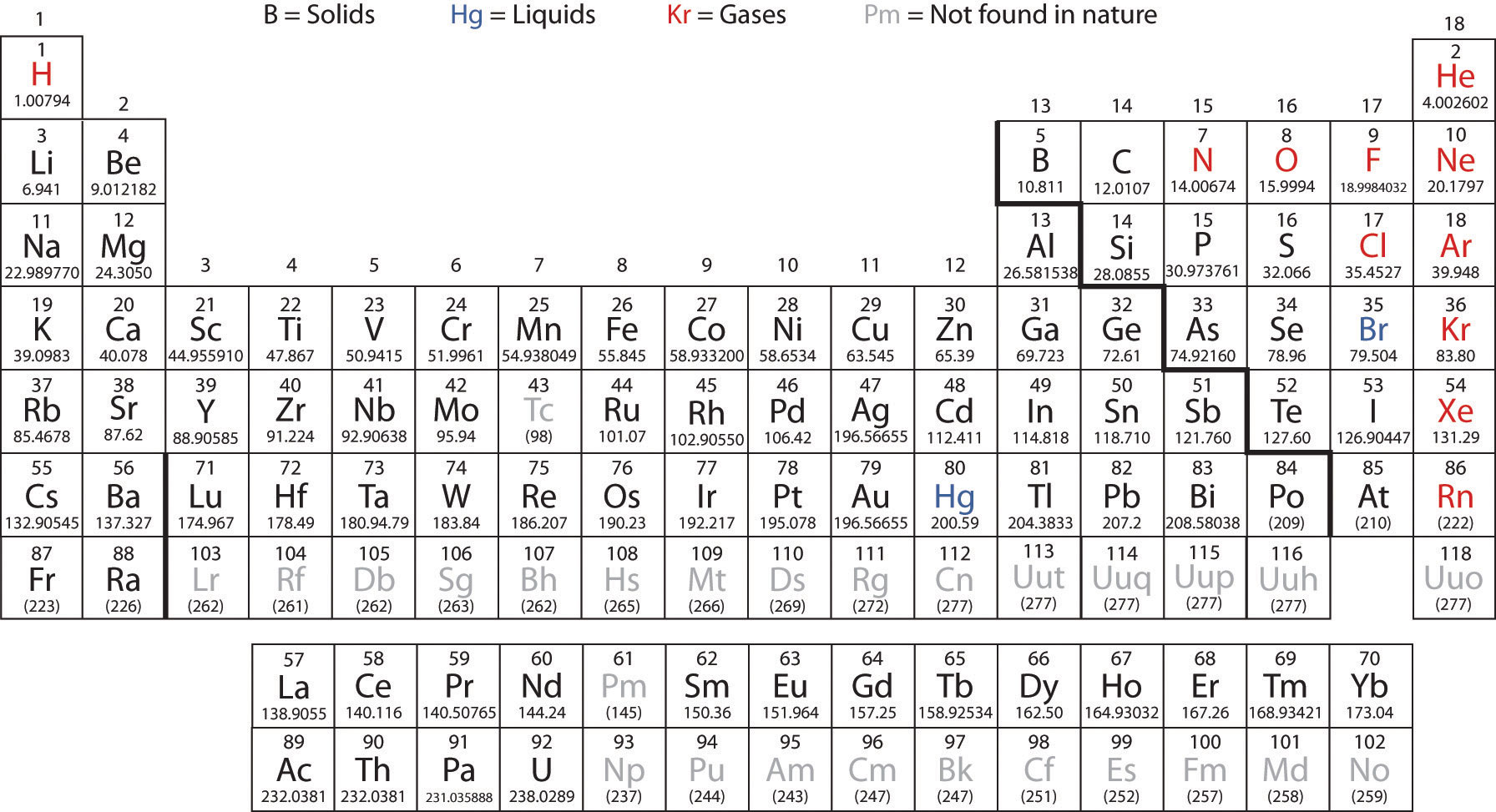
The periodic table was first developed and published by Russian chemist Dmitri Mendeleev, in 1869.

However, in most cases, it is placed above the alkali metal group, being included simply in the non-metal group along with elements like carbon, nitrogen, and oxygen. Due to this, it is sometimes placed separately in the table. Hydrogen is the first element in the periodic table and shows great similarities in properties with both alkali metals and halogens. Six of the groups are numbered, with the elements having their own group names as well, for example, the alkali metals are in Group 1, halogens in Group 17, and noble gases in Group 18. Usually, each row, or period, has the metal elements on the left, while the non-metals are on the right. The rows are known as periods and the columns are known as groups. Similar atomic numbers mean that the elements have similar atomic structure, thus similar chemical properties.


 0 kommentar(er)
0 kommentar(er)
